Background:
Children, adolescents, and young adults (C-AYA) with newly diagnosed advanced Mature B Non-Hodgkin Lymphoma (MB-NHL), specifically Burkitt Lymphoma (BL) and Diffuse Large B Cell Lymphoma (DLBCL), have achieved ≥ 90% overall survival with multi-agent chemotherapy and intrathecal chemotherapy (IT) without central nervous system (CNS) radiation (Cairo et al., Blood 2007; Goldman/Cairo et al., BJH 2014; Frazier/Cairo, et al., BJH 2014; Minard-Colin, et al., NEJM 2020). Standard therapy includes short but intensive courses of multiagent chemotherapy including CNS-penetrating high dose methotrexate and cytarabine for CNS penetration as well as vincristine, prednisone, doxorubicin, and cyclophosphamide with or without etoposide. Patients also receive 9, 10 and 13 IT doses of intrathecal chemotherapy when treated according to FAB Group B, FAB Group C (CNS -) and FAB Group C (CNS +) protocols, respectively. While this approach has effectively eliminated need for CNS radiation for disease control, the need for sedation of patients during these procedures results in the need for repeated episodes of patient fasting, increased healthcare costs, and most importantly, general anesthesia is associated with long-term late effects in cognitive development (Banerjee et al., JAMA Oncol, 2019).
Liposomal cytarabine (LC) is a formulation of cytarabine encapsulated in spherical multivesicular, biodegradable particles known as DepoCyt® which has a maximum-tolerated intrathecal dose of 35 mg in patients between the ages of 3 and 21 years (Bomgaars et al., JCO, 2004). This liposomal formulation has a half-life of 100-263 hours compared with only 3.4 hours of standard cytarabine. As an s-phase specific agent, prolonged exposure may increase its efficacy in controlling CNS disease while decreasing the total number of intraventricular doses required for treatment.
Objective:
To determine the safety and efficacy of reduction of IT therapy and substitution of LC within modified FAB group B and C backbones of systemic immunochemotherapy.
Methods:
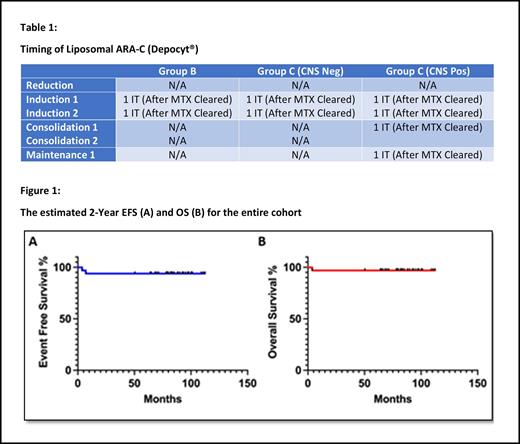
LC was incorporated into the multiagent chemotherapy regimen in a unique manner to mitigate the risk of possible CNS toxicity. A total of 2 doses of LC were administered as part of the prophylaxis IT therapy for Group B and CNS negative group C patients. A total of 4 doses of LC were administered as part of the treatment of Group C CNS + patients (Table 1). The LC was given only in phases of therapy in which no systemic cytarabine was administered. When Methotrexate was given, the LC was only given after clearance. Dexamethasone replaced prednisone for all patients to mitigate potential CNS toxicities from LC.
Results:
This trial enrolled 33 patients with newly diagnosed MB-NHL (25 Group B, 8 Group C with 7 CNS+). Median age was 12 (Range 3-25). Males comprised 64% of patients and 52% had Burkitt Lymphoma/Leukemia. The CNS was involved in 21% of patients, and a total of 78 doses of LC was given. Twenty-six patients received 2 prophylactic doses (25 Group B and 1 Group C CNS negative). Six of seven CNS + patients received all 4 planned doses of LC. The median days from the start of the induction cycles until LC administration (post methotrexate clearance) was 3 days.
Of the 33 patients, only 1 (3%) had a transient Grade 3 adverse event (facial nerve palsy). With dexamethasone prophylaxis, there were minimal adverse events and no toxic deaths reported. There were no other serious adverse events, such as seizure, headaches, nausea, vomiting, fever, somnolence, confusion, or asthenia that have been previously reported. The 2-year EFS and OS of LC when added to an FAB backbone for MB-NHL, was 94.5% and 97.2%, respectively (Figure 1).
Conclusions:
By utilizing dexamethasone prophylaxis and waiting for systemic methotrexate clearance, we were able to demonstrate that IT LC in C-AYAs with MB-NHL who received FAB chemoimmunotherapy with HD MTX was feasible, safe, and did not compromise event free and overall survival. We were also able to reduce the total number of IT injections without apparent compromise to CNS prophylaxis and treatment.
Disclosures
Cairo:Miltenyi Biotec: Research Funding; Sobi: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Amgen Inc.: Honoraria, Speakers Bureau; Servier Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Omeros Pharmaceuticals: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celularity: Research Funding; Astra Zeneca: Honoraria; Merck: Research Funding; Novartis: Consultancy; Abbvie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal